The molar mass of a chemical compound is a crucial concept in chemistry, representing the mass of one mole of that substance. In the case of potassium nitrate (KNO3), calculating its molar mass involves summing the atomic masses of its constituent elements: potassium (K), nitrogen (N), and oxygen (O). The atomic masses are approximately potassium = 39.0983 g/mol, nitrogen = 14.0067 g/mol, and oxygen = 15.9994 g/mol.
Calculation of Molar Mass of KNO3
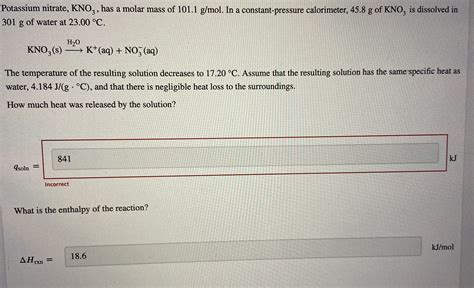
To find the molar mass of KNO3, we add the atomic mass of one potassium atom, one nitrogen atom, and three oxygen atoms together. The formula for this calculation is: Molar mass of KNO3 = atomic mass of K + atomic mass of N + (3 * atomic mass of O).
Substituting the atomic masses into the formula gives: Molar mass of KNO3 = 39.0983 g/mol + 14.0067 g/mol + (3 * 15.9994 g/mol). Performing the calculation: Molar mass of KNO3 = 39.0983 g/mol + 14.0067 g/mol + 47.9982 g/mol.
Summing these values yields the molar mass of KNO3: Molar mass of KNO3 = 101.1032 g/mol. Rounding to the appropriate significant figures based on the input data, the molar mass of KNO3 is approximately 101.10 g/mol.
Significance of Molar Mass in Chemical Reactions
The molar mass of compounds like KNO3 is essential in chemistry for calculating the amounts of substances involved in chemical reactions. It helps in determining the stoichiometry of reactions, which is critical for predicting the quantities of reactants needed and products formed. This is particularly important in industrial processes, where the efficiency and yield of reactions can significantly impact production costs and environmental impact.
Key Points
- The molar mass of KNO3 is calculated by summing the atomic masses of its constituent elements: potassium, nitrogen, and oxygen.
- The atomic masses used are approximately 39.0983 g/mol for potassium, 14.0067 g/mol for nitrogen, and 15.9994 g/mol for oxygen.
- The calculation involves adding the mass of one potassium atom, one nitrogen atom, and three oxygen atoms.
- The molar mass of KNO3 is approximately 101.10 g/mol, which is crucial for stoichiometric calculations in chemical reactions.
- Understanding and applying molar masses are fundamental skills in chemistry, essential for both theoretical studies and practical applications.
| Element | Atomic Mass (g/mol) | Number of Atoms in KNO3 | Total Mass Contribution (g/mol) |
|---|---|---|---|
| Potassium (K) | 39.0983 | 1 | 39.0983 |
| Nitrogen (N) | 14.0067 | 1 | 14.0067 |
| Oxygen (O) | 15.9994 | 3 | 47.9982 |
| Total | 101.1032 |
In conclusion, the molar mass of KNO3, calculated as approximately 101.10 g/mol, is a fundamental piece of information for chemists. It underlines the importance of understanding the composition and properties of chemical compounds, which is vital for advancing research, developing new materials, and optimizing industrial processes.
What is the significance of calculating the molar mass of a compound like KNO3?
+Calculating the molar mass of KNO3 is significant because it allows for the determination of the amount of substance involved in chemical reactions, which is crucial for stoichiometric calculations and predicting reaction outcomes.
How does the molar mass of KNO3 impact its industrial applications?
+The molar mass of KNO3 is essential in industrial applications for calculating the quantities of KNO3 needed for specific reactions, ensuring efficiency, and minimizing waste. This is particularly important in the production of fertilizers, fireworks, and other commodities where KNO3 is a key ingredient.
What are the primary elements that contribute to the molar mass of KNO3?
+The primary elements contributing to the molar mass of KNO3 are potassium (K), nitrogen (N), and oxygen (O), with their respective atomic masses summing up to give the molar mass of KNO3.
